Which Energy Level Holds a Maximum of Eight Electrons
The eight areas include discharge pulse power regenerative pulse power available energy efficiency cycle life system weight system volume and self discharge. There is a formula for obtaining the maximum number of electrons for each shell which is given by 2n2ldots where n is the position of a certain.

How Many Electrons Can The Fourth Energy Level Hold At Level
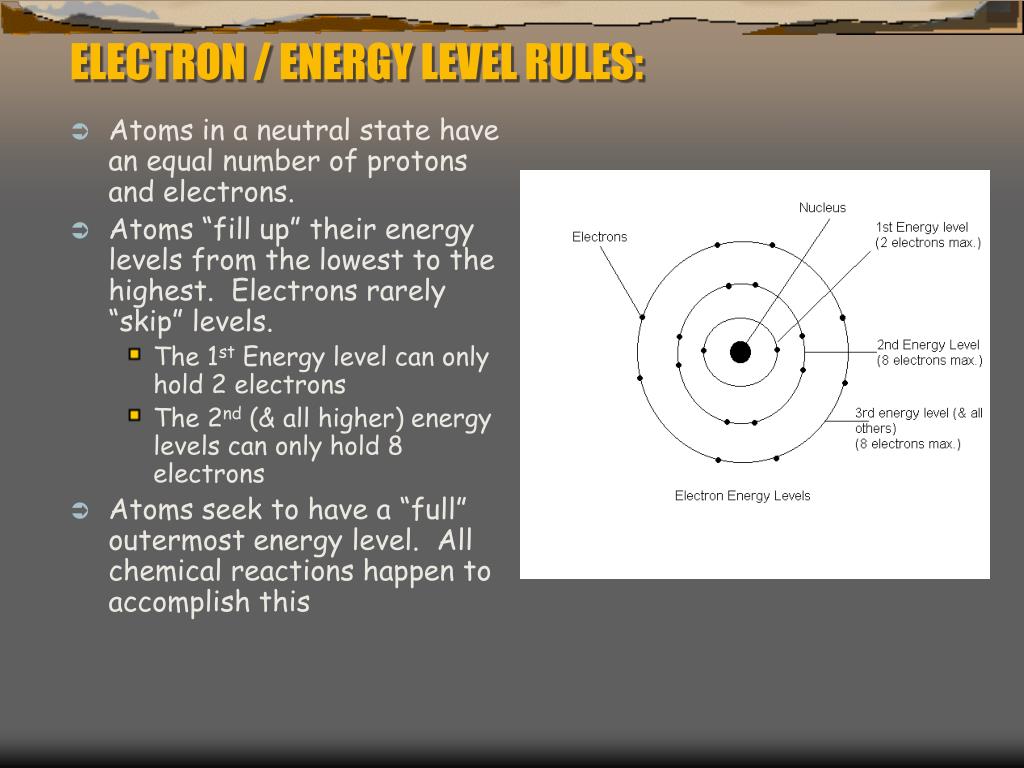
The third shell can carry up 18 electrons but it is more stable by carrying only eight electrons.

. The first shell can carry up to two electrons the second shell can carry up to eight electrons. Still three goals seem to be more challenging and remain unmet. The DOEs short-term goals for power-assist HEVs are met or exceeded in eight of 11 areas showing the tremendous success of the program.

Question Video Finding The Number Of Additional Electrons An Electron Shell Can Hold Nagwa
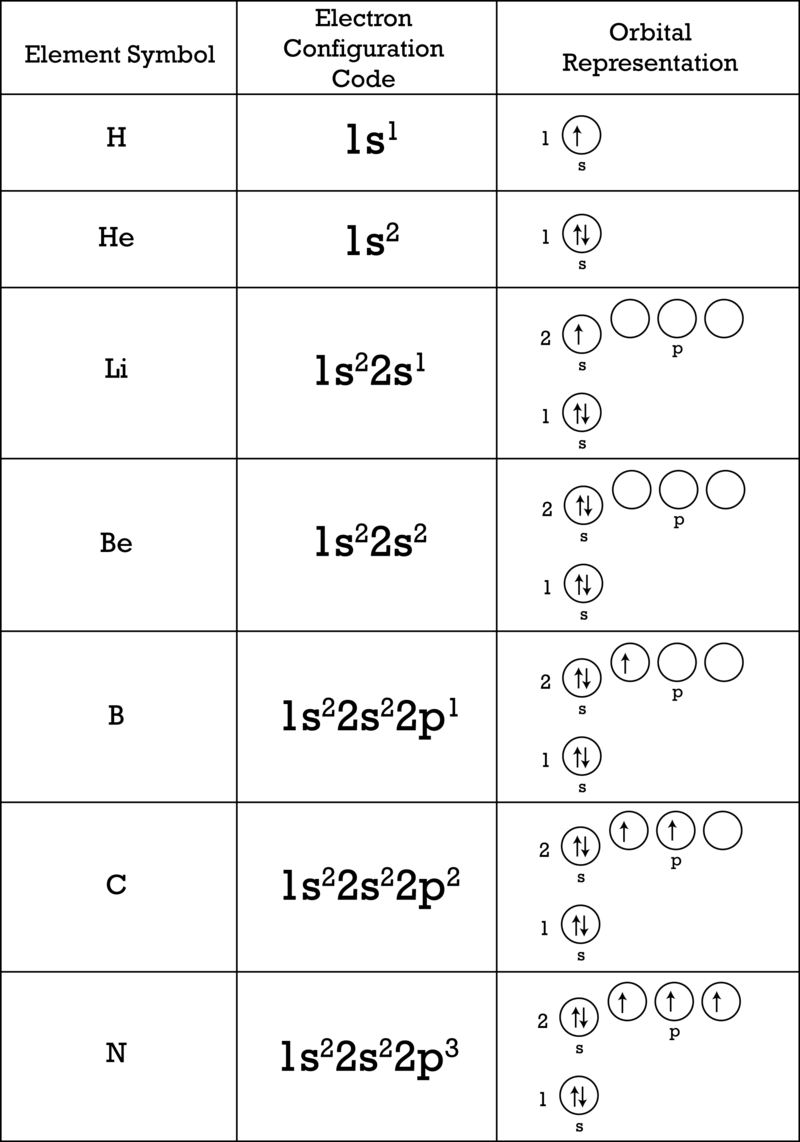
Electron Arrangement Ck 12 Foundation

How Many Electrons Can The 4th Energy Level Hold At Level

Question Video Identifying The Letter That Is Not Used To Represent An Energy Level Nagwa
How Many Electrons Can The Fourth Energy Level Hold At Level

Question Video Determining How Many Electrons Are Needed To Fill Energy Levels K And L Nagwa

How Many Electrons Can The Third Energy Level Hold At Level

Question Video Identifying The Number Of Filled Electron Shells In An Atom Nagwa
How Many Electrons Are In The Highest Occupied Energy Level Of Beryllium Quora

How Many Electrons Are In The Second Energy Level At Level

How Many Electrons Can Fit In The First Energy Level At Level

Question Video Calculating The Number Of Electrons That Must Be Lost For An Atom To Gain A Full Outer Shell Using Its Electronic Configuration Nagwa

How Many Electrons Can Fit In The First Energy Level At Level

How Many Electrons Can The Third Energy Level Hold At Level
How Many Electrons Can The Third Energy Level Hold At Level

Structure And Properties Of Matter Electron Configuration Ppt Download



Comments
Post a Comment